New weight loss medicine seen as an alternative to Ozempic, GLP-1 drugs
Dr. Mahsa Tehrani discusses how patients use medication to help them lose weight on 'America Reports.'
New research has discovered certain weight-loss medications could be associated with an increased risk of serious eye conditions, and even vision loss.
Two studies, published in JAMA, analyzed how semaglutide and tirzepatide — which include popular drugs like Ozempic, Wegovy, Mounjaro and Zepbound — impacted eye health in Americans with type 2 diabetes over a two-year period.
One study found a modest risk of developing non-arteritic anterior ischaemic optic neuropathy (NAOIN) – a rare eye condition that can lead to sudden vision loss due to lack of blood flow – in association with semaglutide and tirzepatide.
Out of more than 159,000 study participants with type 2 diabetes, 35 developed NAION, compared to 19 people in the comparison group.
The Ohio-based researchers also noted an increased risk of developing "other optic nerve disorders," identified in 93 patients.
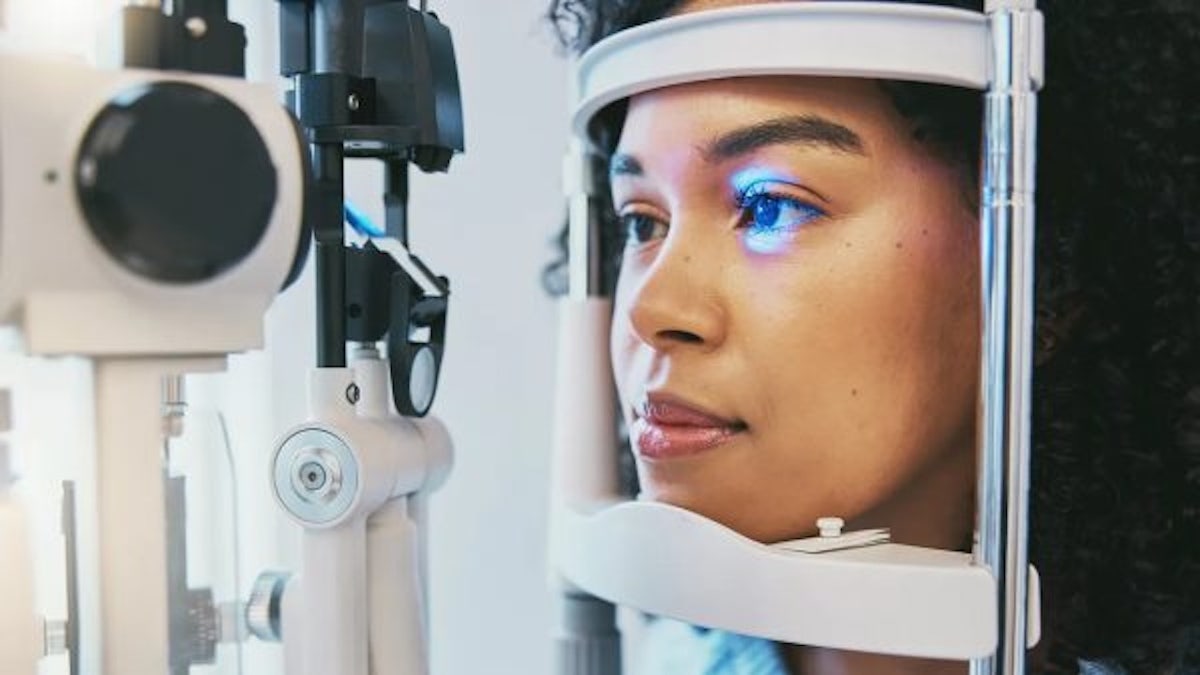
A study discovered that certain GLP-1 drugs are associated with a higher risk of eye conditions. (iStock)
Although the second study did not observe a "statistically significant difference" in NAION in GLP-1 drug users, there was a small increase in diabetic retinopathy, an eye disease that can damage the retina.
DIABETIC PATIENTS TAKING GLP-1S MAY FACE INCREASED RISK OF EYE DISEASE, STUDY SUGGESTS
While individuals with type 2 diabetes on GLP-1s showed a modestly increased risk of diabetic retinopathy, the researchers concluded that fewer patients experienced sight-threatening complications from the disease.
"These findings suggest that all patients with type 2 diabetes treated with GLP-1 RAs, regardless of preexisting diabetic retinopathy, should be regularly screened and monitored for potential complications," the study authors concluded.

Type 2 diabetes patients should be regularly monitored for complications associated with the disease, researchers urge. (iStock)
Sue Decotiis, M.D., a medical weight loss doctor in New York City, said she believes more studies are required to confirm the association between these drugs and vision loss, as these studies report some conflicting results.
"NAION is a rare condition of the optic nerve that, although serious, has not really been shown to be increased by these studies," Decotiis, who was not involved in the research, told Fox News Digital. "We need more studies for certain."
CLICK HERE TO GET THE FOX NEWS APP
Diabetic patients already face a high incidence of eye disease related to blood flow and nerve damage, the expert noted.
"Eye complications are often directly related to the degree or lack thereof of diabetes control."
In most cases, GLP-1 drugs reduce the severity of type 2 diabetes, therefore reducing the incidence of eye diseases, Decotiis noted.
These drugs have also been shown to reduce the risk of cardiovascular disease, like hypertension, and to improve circulation, which can improve eye health.

More research is needed to confirm the link between weight-loss drugs and eye diseases, an expert said. (iStock)
For diabetics who are starting a GLP-1, Decotiis recommends having an exam done by an ophthalmologist and scheduling follow-up exams throughout treatment.
"We should take precaution with methodical ophthalmic care for diabetics on these drugs," Decotiis said. "However, let’s not throw the baby out with the bath water."
Dr. Ashley Brissette, an ophthalmologist in New York City, told Fox News Digital that NAION is an "extremely rare" but serious medical condition.
CLICK HERE TO SIGN UP FOR OUR HEALTH NEWSLETTER
She agreed that the risk with GLP-1 use is also extremely rare, at a risk of about one in 10,000, according to other studies.
"I think caution with anything is warranted," she said. "And remember these are associations, not causations, so it's not to say that GLP-1 use causes NAION or worsening retinopathy, but their use is associated with these conditions."

"These findings underscore the importance of baseline and follow-up eye examinations for patients starting GLP-1 therapy," an ophthalmologist said. (iStock)
"From an ophthalmologic standpoint, these findings underscore the importance of baseline and follow-up eye examinations for patients starting GLP-1 therapy, especially those with pre-existing retinal or optic nerve risk factors."
The expert added that while the benefits of GLP-1 medications in reducing cardiovascular and metabolic risks are "substantial," patients should be counseled on potential visual side effects, and "any sudden visual symptoms should prompt immediate ophthalmic referral."
For more Health articles, visit www.foxnews.com/health
Novo Nordisk, maker of Ozempic and Wegovy, provided the following statement when contacted by Fox News Digital.
"Patient safety is a top priority for Novo Nordisk, and we take all reports about adverse events from the use of our medicines very seriously. NAION is a very rare eye disease, and it is not an adverse drug reaction for the marketed formulations of semaglutide (Ozempic, Rybelsus and Wegovy) as per the approved labels in the U.S."
"Novo Nordisk, on its part, has conducted an analysis across randomized controlled clinical trials with GLP-1 receptor agonists, including a blinded ophthalmologist evaluation to confirm NAION diagnoses. Our current assessment is that these data do not suggest a causal relationship between GLP-1 RA use and NAION events."











































